Warning: The information provided here is for educational purposes only and should not be considered accurate. The use of this information to cause harm to another individual is in no way condoned by the author and is a serious criminal offense. If you are reading this article with a view to self harm or suicide, please seek immediate professional help.
How dangerous is dangerous?
 DMSO, or dimethylsulfoxide, is one of those chemicals in the laboratory that actually scares me since, in itself, it’s not obviously that dangerous: the risk phrases in the health and safety data just list it as irritating to the eyes, respiratory system and skin, which is pretty much an everyday event in the lab of a synthetic chemist. The safety information isn’t much different: it advises you to wear gloves and safety goggles when using it, and if you do get it in your eyes then to wash them out with water. No real surprises there.
DMSO, or dimethylsulfoxide, is one of those chemicals in the laboratory that actually scares me since, in itself, it’s not obviously that dangerous: the risk phrases in the health and safety data just list it as irritating to the eyes, respiratory system and skin, which is pretty much an everyday event in the lab of a synthetic chemist. The safety information isn’t much different: it advises you to wear gloves and safety goggles when using it, and if you do get it in your eyes then to wash them out with water. No real surprises there.
To keep this in perspective, if you look up the safety information for water, it too recommends wearing safety goggles. It even goes on to suggest that if you get it in your eyes, you need to wash them out with water (presumably from the eye wash station, rather than the stuff you just got in your eyes).
Measuring toxicity
A convenient way to measure the toxicity of a chemical is to calculate its LD50 (median lethal dose). This is the amount of substance that, if given to an individual, they would have a 50:50 chance of living. While there are many limitations with these values, for example varying methods of administration, it provides a good guideline. In addition, as animals don’t come in uniform shapes and sizes, these are often quoted per kilogram weight of a typical animal, and rats are generally used over human test subjects for obvious reasons. However, it is important to take into account that the LD50 is only a measure of the median dosage; some unlucky individuals will not survive much lower doses than that.
As an example, the LD50 for the oral administration of water to rats is 90 g/kg. Assuming that rats and humans are not too dissimilar in their metabolism of water, the equivalent would be for you to attempt to down 8 litres of water (the same as four large bottles of cola). Obviously, there is a far greater risk of drowning in 8 litres of water than the accidental self-administration of it orally (which still probably holds a significant risk of drowning before consuming the entire volume). Yet the point stands: in cases when large amounts of water are consumed, typically highlighted in the media as a side effect of recreational drug use, the results for the individual can be fatal as the brain swells, which pushes on the skull, causing death.
To compare, DMSO has an oral LD50 in rats of 15 g/kg, which is about 1.2 litres when applied to an adult human. Now I don’t advise placing bets on these numbers (unless you are a rat that is fully literate in English – if so, please do leave a comment on how to contact you). There is a large chance these numbers are way off.
The table below contains a rough list of the oral LD50 of some standard toxins:
| Toxin | LD50 (g/kg) |
| Botulinium toxin | 0.00000005 |
| Tetrodotoxin (Fugu/blowfish toxin) | 0.0003 |
| Hydrogen cyanide (gas) | 0.001 |
| Potassium cyanide (solid) | 0.2 |
| Hemlock | 1.7 |
| Methanol | 6 |
| Potassium ferrocyanide | 6.4 |
| Ethanol | 10 |
| DMSO | 15 |
| Water | 90 |
It’s not just content, delivery is important too
Interestingly, potassium ferrocyanide is a poor poison yet was once part of an alleged terrorist attack attempt on the US Embassy in Rome, Italy in 2002: the terrorists’ plan was to place 9 lb of the compound into the Embassy’s water supply. The reality, though, is that it certainly would not have been enough to make it toxic: the chemistry behind potassium ferrocyanide is that iron in ferrocyanide tightly co-ordinates the cyanide anion around itself, and the acid in the stomach is unable to displace it from the iron (the ferrocyanide presumably then passes through the body and is excreted as waste).
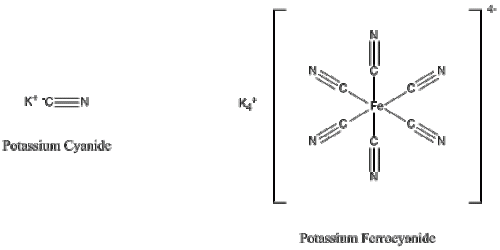
While potassium ferrocyanide contains more of the toxic cyanide anion, the tight binding of it to the iron (Fe) centre reduces its ability to be displaced by stomach acid.
As for Agatha Christie’s weapon of choice, potassium cyanide, however, the LD50 for our hypothetical human would be 15-20 g, roughly two and a half teaspoons. This is not an insignificant amount to ingest by mistake. Admittedly, it’s much less than the 8 litres required for water intoxication, but within a lab the risk involved in its use is low if the right precautions are taken. On the other hand, a would-be assassin would have little trouble administering the material since potassium cyanide is very soluble in water: 70 g will dissolve into 100 mL of water at room temperate (a cup of tea by comparison is about 230 mL and its raised temperature makes the poison even more soluble).
Whereas ferrocyanide is relatively inert, potassium cyanide administered to the victim via a fatal cup of Earl Grey tea would react to form hydrogen cyanide on contact with stomach acid. This is due to the cyanide anion not being as tightly bound to the potassium as it is to the iron in ferrocyanide. The cyanide anion is then transported into the body, where it binds to the iron within an enzyme called cytochrome c oxidase. The ability of the cytochrome c oxidase to bind with oxygen is consequently disrupted, a vital step in the chain of reactions required for our cells to convert food and oxygen into energy, and thus parts of the body that are particularly dependent on this start to fail, particularly the central nervous system and the heart.
Water off a duck’s back
Oil and water don’t mix. In chemistry, we divide these liquids or solvents up into the categories of polar and non-polar. Water is a polar solvent: each molecule, while not carrying a charge itself, has a partial positive charge on the two hydrogen atoms and a partial negative charge on the oxygen, hence we call this a dipole. This, in part, results in water molecules sticking together strongly (and why it has a high boiling point for a liquid) as the partial negative charge on one molecule of water is attracted to the partial positive charge on one of the next molecule’s hydrogen atoms. It also means that it dissolves ionic compounds (that is compounds made up of positive and negative ions, such as sodium chloride/sea salt) very well as it can stabilise the positive charge of the sodium cations and the negative charge of the chloride anions floating around in solution.
On the other hand, we have the non-polar solvents, such as hexane or chloroform. These are very poor at dissolving salt as they do not have the ability to stabilise the ions in solution like water. Therefore, the ions in salt would rather bind together and stay as a solid. The converse is also true: water is poor at dissolving compounds that are not charged or polar as it would far rather stick to other water molecules, and so pushes the compound out of solution.
DMSO, while being a polar solvent like water, has an unusual ability to mix with a wide range of both polar and non-polar solvents. In addition, it is pretty much able to dissolve nearly anything. These properties, combined with the fact that it is fairly inert to reactions, make it almost a perfect solvent to conduct chemical reactions in. Well, that is until you want to get the product out of solution, at which point you start cursing it for being such a good solvent.
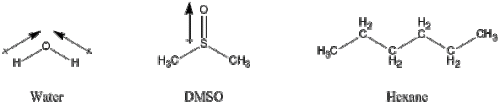
Water and DMSO are examples of polar solvents while hexane is non-polar. The arrows show the polarisation of the bonds, pointing from the partial positive charge to the partial negative one. Together, this pairing of positive and negative charges is known as a dipole.
Only skin deep
The fact that DMSO is a fantastic solvent means that it can be a nightmare in the lab, but the problems don’t stop there. Due to its ability to mix with such a wide range of solvents, it can do something most compounds won’t: it can efficiently and quickly penetrate our skin. Apparently, after contact with the skin, it is possible to taste it minutes later. I’ve never tested this anecdote, and you’ll forgive me if I don’t recommend anyone else attempt to confirm it.
For most compounds, the LD50 for oral and skin toxicity are widely different. Skin has evolved as an effective barrier against the environment and, if not damaged, works well at protecting us. Yet despite this, DMSO’s impressive miscibility allows it to bypass this defense. Combined with its near universal ability to dissolve anything in the lab (including gloves), this has terrifying consequences as it will then go on and carry potentially more harmful toxins into your body.
The result is obvious: combining DMSO with the wrong compound will rapidly increase the risk of the situation and, if you’re unlucky, the results could be fatal. One such compound DMSO will dissolve is potassium cyanide, making it a liquid that will poison you on contact with the skin or through your gloves. It is not surprising that this mixture of properties has earned it the reputation of ‘Liquid Death’.
I can verify your story – having used DMSO within seconds of getting it on your skin, an odd taste of slightly rotten cabbages is experienced!
DMSO will drag anything across the skin and is used fir this purpose by some drugtakers
T
The cabbage taste would make sense as most DMSO contains dimethylsulphide as an impurity which has a cabbage smell. The other options is the body converts if from DMSO to DMS which I’ve saw some evidence for in the literature when looking up its toxicity.
Hey, I noticed the Botulinium Toxin is top on this list, but how come people get Botox Shots and don’t die from it?
People do die from (badly administered) Botox Shots, the dose is key, and very very low. From memory it blocks nerve signals, you paralyse you face to make it look better. You can image that if you don’t localise the effect it’s going to kill very quickly.
I notice you make absolutely no mention that a 50% solution of DMSO is the FDA-approved treatment for interstitial cystitis.
There are plenty of things I wasn’t able to include, the piece was much longer than I originally intended. I don’t know much about DMSO’s use for cystitis so wouldn’t want to comment if it’s good or bad. The fact the FDA have approved it gives some weight to it being effective, but I’d need to check the primary literature before being taking a side. Cystitis is one of those things that as far as I am aware is a real problem for some women and treatments generally are not that affective. That always makes me want to check it isn’t just a placebo before giving my two cents.
So my answer basically no comment as I’m not informed enough to make one. I hope you appreciate honesty.
Hello,
I ran into your post when searching for the safety of Ferric Ferrocyanide. I bought quite a few eyeshadows that contain it and I didn’t know till I got home. I even tested two of them already and they’re on my eye lids. What are your thoughts? Am I safe using them? I just hope they don’t cause blindness, heh. Thanks!
These kind of cyanide compounds are typically safe for the reasons I commented in the article. There’re very stable and very unlikely to release active cyanide in a toxic form.
So I wouldn’t worry about it. You’ll find it in shampoo and dish washer products.
Very informative, thank you very much! Phewf!